Resolution Therapy

Resolution Pharmacology: State-of-the-art and therapeutic landscape
Mauro Perretti, Trinidad Montero-Melendez
The process of the resolution of inflammation represents an integral part of the whole acute inflammatory response. Dysregulation in resolution mechanisms can lead to disease. Conversely, harnessing resolution can offer therapeutic guidance to develop medicines that are disease independent, broadening their potential. There is ongoing intensive clinical development in this area. Pro-resolving drugs will be patient centric in their pharmacology and would promote natural processes of healing and repair.
Resomelagon (AP-1189) in Rheumatoid Arthritis

AP1189: a novel, oral, biased melanocortin agonist with anti-inflammatory and pro-resolving effect for the treatment of rheumatoid arthritis
AP1189 is a first-in-class novel, orally available compound with an anti-inflammatory effect and good tolerability profile and pharmacokinetic properties allowing for once-daily dosing, with the potential to treat active RA and other serious diseases associated with hyper-inflammation.
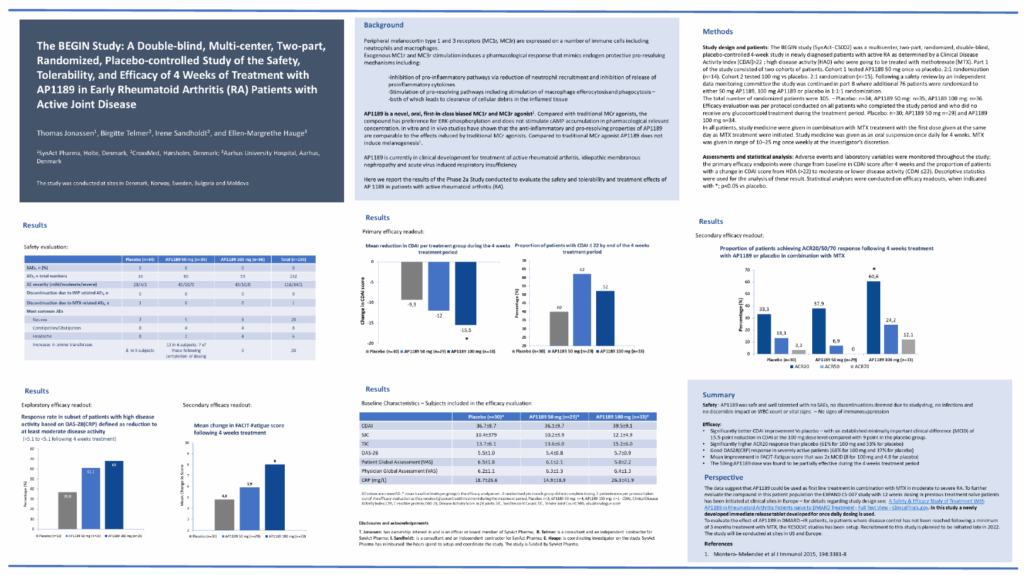
The BEGIN Study: A Double-blind, Multi-center, Two-part, Randomized, Placebo-controlled Study of the Safety, Tolerability, and Efficacy of 4 Weeks of Treatment with AP1189 in Early Rheumatoid Arthritis (RA) Patients with Active Joint Disease
AP1189 was safe and well tolerated with no SAEs, no discontinuations deemed due to study drug, no infections and no discernible impact on WBC count or vital signs — No signs of immunosuppression
Significantly better CDAI improvement vs placebo — with an established minimally important clinical difference (MCID) of 15.5-point reduction in CDAI at the 100 mg dose level compared with 9 point in the placebo group. Significantly higher ACR20 response than placebo (61% for 100 mg and 33% for placebo). Good DAS28(CRP) response in severely active patients (68% for 100 mg and 37% for placebo).
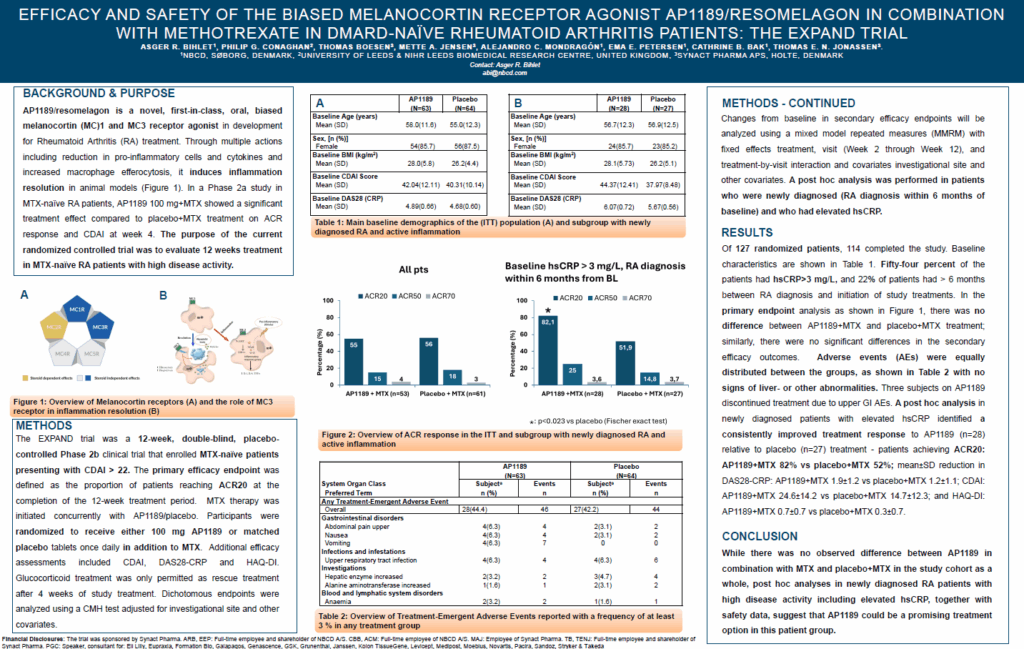
The EXPAND study: Efficacy and Safety of the Biased Melancortin Receptor Agonist AP1189/Resomelagon in Combination with Methotrexate in DMARD-Naïve Rheumatoid Arthritis Patients
AP1189/resomelagon is a novel, first-in-class, oral, biased melanocortin MC1 and MC3 receptor agonist in development for Rheumatoid Arthritis (RA) treatment. Through multiple actions including reduction in pro-inflammatory cells and cytokines and increased macrophage efferocytosis, it induces inflammation resolution in animal models. The purpose of the current randomized controlled trial was to evaluate 12 weeks treatment in MTX-naïve RA patients with high disease activity.
Resomelagon (AP-1189) in Viral Infections

Effects of a pro-resolving drug in COVID-19: preclinical studies to a randomized, placebo-controlled, phase Ib/IIa trial in hospitalized patients
Pedro R. J. Almeida et al.
Treatment with AP1189 attenuated pulmonary inflammation in mice infected with MHV-A59 or SARS-CoV-2 and decreased the release of CXCL10, TNF-α and IL-1β by human PBMCs. Hospitalized COVID-19 patients already taking glucocorticoids took a median time of 6 days until respiratory recovery when given placebo versus 4 days when taking AP1189 (P = 0.017).