Phase 2B 12-week study in early DMARD naïve RA-participants with high disease activity and active inflammation in combination with MTX (ongoing)
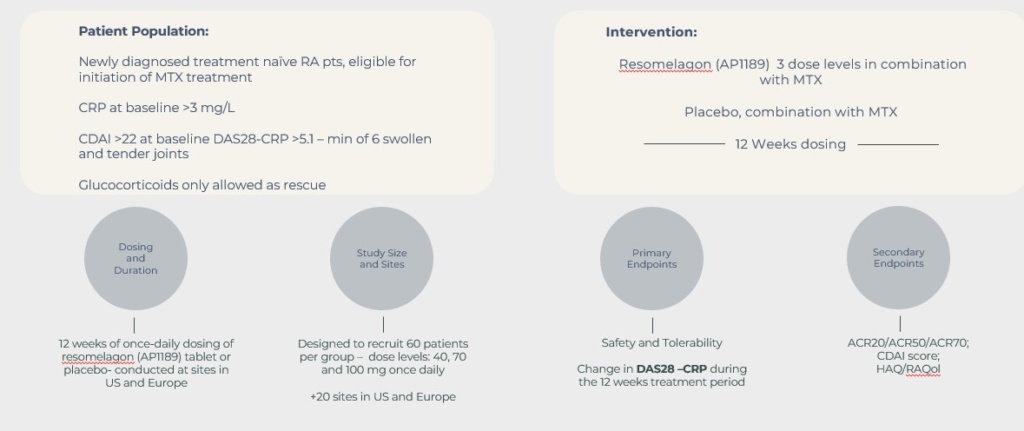
Based on knowledge of resomelaon in RA from previous clinical trials, the new ADVANCE study is a phase 2B proof of concept study in the target population for resomelagon, i.e. newly diagnosed RA-patients with high disease activity including signs of systemic inflammation where there is unmet medical need for a safe and effective convenient oral treatment with the potential in combination with the first line compound MTX to increase the likelihood of disease control with reduced use of glucocorticoid and when the potential to postpone the use of second treatments as the TNF-blockers.
The ADVANCE study is a randomized, double blind, placebo-controlled, dose response, phase IIb, multicenter trial to evaluate the efficacy and safety of once daily oral resomelagon (AP1189) administered at the doses of 40, 70, or 100 mg for 12 weeks in combination with MTX, in DMARD-naïve newly diagnosed RA-patients with high disease activity and signs of systemic inflammation.
The aim is to recruit a total of 240 patients with a reduction in DAS28- CRP as the primary efficacy readout and will be conducted as an international study under the current US-IND (FDA) for development of resomelagon (AP1189) in RA.
The ADVANCE is ongoing in several European countries as well as in the USA. The recruitment is according to plan and currently the safety profile is in accordance with earlier studies.