Phase 2A in early severe RA together with MTX (finalized)
The BEGIN study in early severe RA was completed in 2021. The study was a randomized, double-blind, placebo controlled multicenter study in previous treatment naïve RA patients where either 50 mg or 100 mg of resomelagon or placebo were given in addition to MTX treatment.
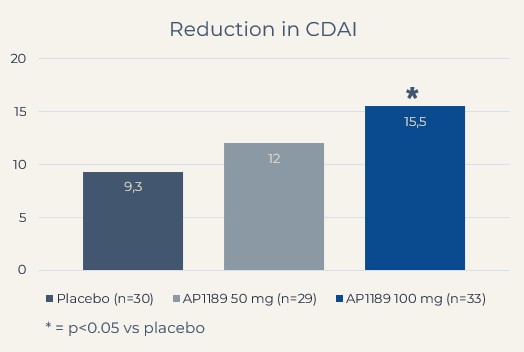
100 mg of resomelagon (AP1189) demonstrated a statistically significant mean reduction in the clinical disease activity index (CDAI), the primary study endpoint, from baseline to four weeks that was more than 65% higher than the effect seen in the placebo-treated control group (mean reduction in CDAI: resomelagon (AP1189) 100 mg (n=33): 15.5 points compared with placebo (n=30): 9.3%, p = 0.0394).
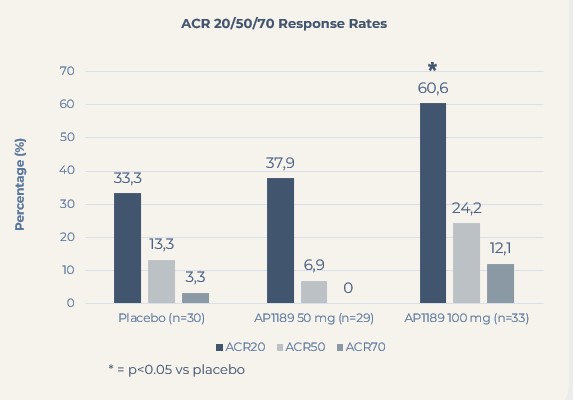
Resomelagon given once daily for four weeks was safe and well tolerated. Based on the primary read out, changes in clinical disease activity index (CDAI), the data showed a clear dose response for 50 and 100 mg resomelagon relative to placebo, with 100 mg of resomelagon demonstrating a statistically significant 65% higher mean reduction in CDAI during the treatment period compared to placebo-treated control group (mean reduction in CDAI: resomelagon 100 mg (n=33): 15.5 points compared with placebo (n=30): 9.3 points, p = 0.0394). The 100 mg resomelagon group also demonstrated a significantly higher fraction of patients achieving ACR20 than placebo treated patients (ACR20: resomelagon (n=33) 100 mg: 60.6%; Placebo (n=30): 33.3%, P=0.0437) within the 4 weeks treatment period.